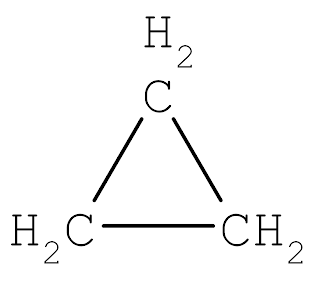
 |
| Cyclopropane |
When naming the compound, you can start from carbon atom and go clockwise or counter-clockwise. If there is only one side group, you do not need to put a number because we assume it starts at the very first carbon. When there is more than one side group, count clockwise or counter-clockwise from the first side group so that the lowest numbers are used.
 |
| cyclohexanone |
If there is a tie, we always sort them by alphabetical order.
Alkenes and Alkynes do not need numbers unless there is more than one double/triple bond because we always assume the bond starts from the first carbon.
Aromatics:
They usually have pleasant odours and contains at least one benzene ring (C6H6) which is a cyclohydrocarbon with 3 double bonds between carbon atoms.
The double bonds are delocalized so they can go anywhere and they are less reactive than cycloalkenes and cycloalpynes because of this.
 |
| Benzene |
If the benzene is a side group, we call it phenyl.
This video will sum up what we learned and is a great study guide for the test~! Enjoy

No comments:
Post a Comment