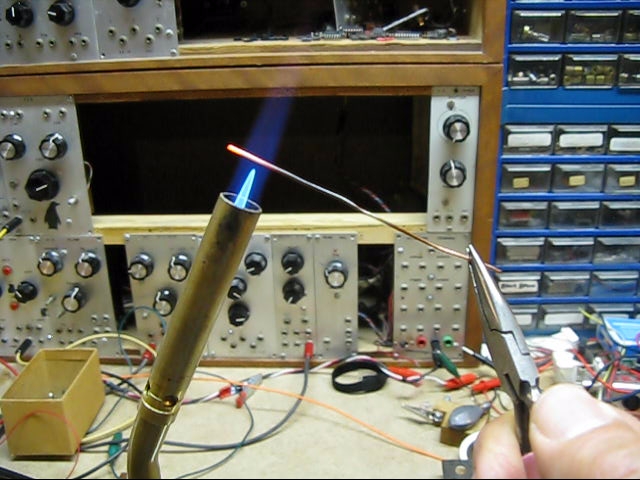
After learning about different types of reactions, we will be experimenting them in this lab. After making observations, we will write down each reaction and classify them.
Objectives
1. Observe reactions (observations help to determine weather a reaction has taken place)
2. Interpret and explain reactions
3. Classify reactions
Procedure (summary)
Reaction 1
Heat a copper wire
2Cu + O2 -> 2CuO Synthesis
Reaction 2
Place an iron nail in copper (II) sulfate
2Fe + 3CuSO4 -> Fe2(SO4)3 + 3Cu Single Replacement
Reaction 3
Heat copper (II) sulfate pentahydrate
CuSO4 5H2O -> CuSO4 + 5H2O Decomposition
Reaction 4
Add water to result of reaction 3
CuSO4 + 5H2O -> CuSO4 5H2O Synthesis
Reaction 5
Pour calcium chloride into sodium carbonate
CaCl2 + Na2CO3 -> CaCO3 + 2NaCl Double Replacement
Reaction 6
Add hydrochloric acid to mossy zinc
2HCl + Zn -> Zn(Cl)2 + H2 Single Replacement
Reaction 7
Add manganese (IV) oxide to hydrogen peroxide
2H2O2 -> 2H2O + 1O2 Decomposition
Jan 26, 2012
Jan 18, 2012
Types of Reactions
A chemical reaction is when one or more substances are changing in to different substances. Typical chemical reactions include burning, decay, fermentation, corrosion of steel and digestion of food.
There are six common types of reactions: Synthesis, Decomposition, Single Replacement, Double Replacement, Combustion, and Neutralization.
Synthesis
BC→B+C
Single Replacement (displacement)
*Predicting Single Replacement Reactions
 1NaCl + Br2
1NaCl + Br2
On the Activity Series, Bromine (Which replaces the Chlorine) is located higher than Chlorine. Therefore, this reaction can be performed.
Double Replacement
 *Using "Table of Solubilities"
*Using "Table of Solubilities"
*NET Equation
Combustion
Neutralization
There are six common types of reactions: Synthesis, Decomposition, Single Replacement, Double Replacement, Combustion, and Neutralization.
Synthesis
- A reaction that combines two or more reactants to form one product.
- General Formula: A+B→AB
- Eg. 2Fe + 1O₂ → 2FeO
BC→B+C
- Eg. 2Ag₂O→4Ag+1O₂
Single Replacement (displacement)
- A reaction where an ionic element replace positive ions and non-metal elements replace negative ions.
- General Formula:
- Eg. 2CuO+1C→2Cu+1CO₂
*Predicting Single Replacement Reactions
- sometimes, single replacement can not be performed because some metals are more reactive than others and so some metals.
- to check if the reaction is possible to happen, use the "ACTIVITY SERIES"ーAn element higher up on the series replaces the ion below it on the table.

On the Activity Series, Bromine (Which replaces the Chlorine) is located higher than Chlorine. Therefore, this reaction can be performed.
Double Replacement
- a reaction between two ionic compounds usually in solution.
- the ions switch partners
- General Formula: AB+CD→CB+AD
- Eg.1Na2CO3 +1CaCl2 → 1CaCO3 + 2NaCl
 *Using "Table of Solubilities"
*Using "Table of Solubilities"- you can check if the reactions of double replacement occurs by determining the states-(aq) or (s) from using the "Table of solubilities"
- If the reactants change state during the reaction, there is a reaction.
*NET Equation
- it is a chemical equation showing only the elements that participates in reaction.
- Eg. 1CuCl2(aq)+ 2NaOH (aq)→1Cu(OH)2(s) + 2NaCl(aq)
Combustion
- a reaction where burning in air is involved.
- the oxygen atoms usually combine with more than one type of atom as products.
- General Formula: AB+O₂→AO+BO
- Eg. 1C3H8 + 5O2 → 3CO2 + 4H2O
Neutralization
- it is one kind of rdouble replacement reaction where acids react with bases to produce water and an ionic salt as product
- General Formula: HA+BOH→H₂O+BA
- Eg.1HCl + 1NaOH → 1NaCl + 1H2O
Jan 16, 2012
Naming and Balancing Compounds
Balancing Equation~~
We want the number of reactant atoms to match the number of atoms on the product side.
Here are some rules that can help you when balancing equations!
1. Balance the atoms that occur in only one molecule
2. Balance whole groups of atoms (polyatomic atoms)
3. Do not jump from one element to another without fully balancing it first
4. Balance atoms that do not combine with others last ( such as S8, O2, or P4)
Example:
S8 + O2----> SO3
Because there are 8 sulfur atoms in the reactant side, the coefficient infront of SO3 should be 8.
S8 + O2 ----> 8SO3
8x3=24, since there are 24 oxide atoms on the product side, the coefficient infront of O2 should be 12.
S8 + 12O2 ----> 8SO3
Lastly, REMEBER TO PUT THE 1 INFRONT OF S8.
the balanced equation is:
1S8 + 12O2 ----> 8SO3
Here is a great link to a tutoral that will help you practice your balancing equations knowledge. It is very interactive!
Naming compounds:
Ionic compounds: name the metal and drop the ending of the non-metal and add "ide"
Li2O = lithium oxide
Polyatomics: Name the metal, name the polyatomic ion
KNO3 = potassium nitrate
Multiple Charges: Name the metal, write its charge in roman numerals in brackets, and then name the non-metal with "ide" at the end
PbO2 = Lead (IV) oxide
Covalent compounds:
write the prefixes infront of the element names and change the ending of the second non-metal to end in "ide"
Note: if the first element in the compound is one, do not at mono infront of it.
example: CO2 = carbon dioxide
Acids:
Simple Acids: HF = Hydrofluoric acid
write hydro for the hydrogen atom and write out flourine but change the ending to "ic acid"
Complex Acid: HNO3 = Nitric acid
drop the hydro and change the ending to "ic acid" if the polyatomic ion ends in "ate"
H2SO3 = Sulfurous Acid
drop the hydro, change the ending to "ous acid" if the polyatomic ends in "ite"
We want the number of reactant atoms to match the number of atoms on the product side.
Here are some rules that can help you when balancing equations!
1. Balance the atoms that occur in only one molecule
2. Balance whole groups of atoms (polyatomic atoms)
3. Do not jump from one element to another without fully balancing it first
4. Balance atoms that do not combine with others last ( such as S8, O2, or P4)
Example:
S8 + O2----> SO3
Because there are 8 sulfur atoms in the reactant side, the coefficient infront of SO3 should be 8.
S8 + O2 ----> 8SO3
8x3=24, since there are 24 oxide atoms on the product side, the coefficient infront of O2 should be 12.
S8 + 12O2 ----> 8SO3
Lastly, REMEBER TO PUT THE 1 INFRONT OF S8.
the balanced equation is:
1S8 + 12O2 ----> 8SO3
Here is a great link to a tutoral that will help you practice your balancing equations knowledge. It is very interactive!
Naming compounds:
Ionic compounds: name the metal and drop the ending of the non-metal and add "ide"
Li2O = lithium oxide
Polyatomics: Name the metal, name the polyatomic ion
KNO3 = potassium nitrate
Multiple Charges: Name the metal, write its charge in roman numerals in brackets, and then name the non-metal with "ide" at the end
PbO2 = Lead (IV) oxide
Covalent compounds:
write the prefixes infront of the element names and change the ending of the second non-metal to end in "ide"
Note: if the first element in the compound is one, do not at mono infront of it.
example: CO2 = carbon dioxide
 |
| prefixes |
Simple Acids: HF = Hydrofluoric acid
write hydro for the hydrogen atom and write out flourine but change the ending to "ic acid"
Complex Acid: HNO3 = Nitric acid
drop the hydro and change the ending to "ic acid" if the polyatomic ion ends in "ate"
H2SO3 = Sulfurous Acid
drop the hydro, change the ending to "ous acid" if the polyatomic ends in "ite"
This video will explain the differences when naming acids in detail.
Jan 6, 2012
Volume Occupied By Gases
Gases will expand or contract depending on the different temperatures and pressure therefore changing the volume of these cases
The standard condition of temperature and pressure is STP and we can use this condition to compare the different volumes of gases
STP = 1 atmosphere of pressure and a temperature of 0 degrees Celcius
At STP, 1 mole of a gas will have a volume of 22.4 Litres
The unit conversion can then be formed as:
Examples:
Remember to count the amount of significant figures when rounding your final answer~!
This video includes some examples as well as higher level questions relating to the concept of volume of gases per mole.
The standard condition of temperature and pressure is STP and we can use this condition to compare the different volumes of gases
STP = 1 atmosphere of pressure and a temperature of 0 degrees Celcius
At STP, 1 mole of a gas will have a volume of 22.4 Litres
The unit conversion can then be formed as:
22.4 L of Gas/ 1 mole of Gas or 1 mole of Gas/22.4 L of Gas
Examples:
2.5 moles CO2 x 22.4 L of Gas = 56 L
Calculate the amound of of volume occupied by 36.0g of NH3.
N= 14.0g H= 1.0g
36g NH3/17.0g = 2.12 moles NH3
2.12 moles NH3 x 22.4L = 47.4 L
This video includes some examples as well as higher level questions relating to the concept of volume of gases per mole.
Jan 4, 2012
Diluting of Solutions
Purpose: To be able to make solutions less concentrated from a more concentrated solution.
Equation
M1L1 = M2L2 , where M1 represents molarity and L1 represents volume before the solute is diluted into less concentrated. The moles of solute must be the same. Only the less concentrated solution contains more water.
Example:
Concentrated NaCl is 10.2 mole/L. How would you make up 300 mL of 0.620 mole/L HCL ?
L1 is unknown. So we rearrange the equation above. You get:
L1 = M2 X L2 / M1 = 0.620 mole/L X 300 mL / 10.2 mole/L
= 18.23529 L
* note the sig fig.
Answer = 20 L
Then, convert 300 mL into L, which is 0.300 L. To calculate how much water is added to the solute,
20 - 0.300 = 19.7 L
To get more practice, here is a worksheet with answers to the questions!
Hope this helps~!
Subscribe to:
Posts (Atom)