After learning about different types of reactions, we will be experimenting them in this lab. After making observations, we will write down each reaction and classify them.
Objectives
1. Observe reactions (observations help to determine weather a reaction has taken place)
2. Interpret and explain reactions
3. Classify reactions
Procedure (summary)
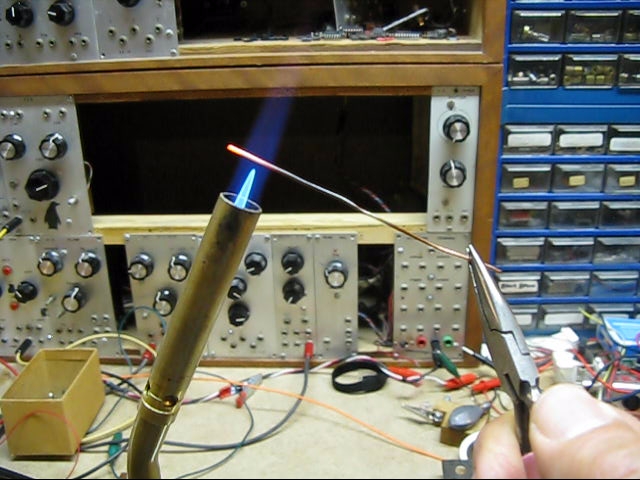
Reaction 1
Heat a copper wire
2Cu + O2 -> 2CuO Synthesis
Reaction 2
Place an iron nail in copper (II) sulfate
2Fe + 3CuSO4 -> Fe2(SO4)3 + 3Cu Single Replacement
Reaction 3
Heat copper (II) sulfate pentahydrate
CuSO4 5H2O -> CuSO4 + 5H2O Decomposition
Reaction 4
Add water to result of reaction 3
CuSO4 + 5H2O -> CuSO4 5H2O Synthesis
Reaction 5
Pour calcium chloride into sodium carbonate
CaCl2 + Na2CO3 -> CaCO3 + 2NaCl Double Replacement
Reaction 6
Add hydrochloric acid to mossy zinc
2HCl + Zn -> Zn(Cl)2 + H2 Single Replacement
Reaction 7
Add manganese (IV) oxide to hydrogen peroxide
2H2O2 -> 2H2O + 1O2 Decomposition





No comments:
Post a Comment